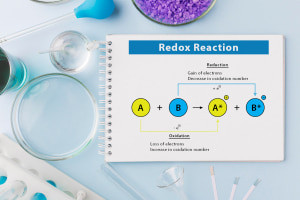
This course begins with an overview of the five types of chemical reactions: combination, decomposition, single-displacement, double-displacement and combustion. We describe each of these reactions in detail to explain how their reactants combine to form one new compound or degrade into more substances. We then cover oxidation numbers and their role in a chemical reaction as atoms or ions lose or gain electrons and are labelled as ‘oxidised’ or ‘reduced’ respectively. The course outlines how these oxidation rules apply to metal and non-metal compounds. Our next topic discusses redox reactions, which involve the exchange of electrons between reactants. We show you how to name inorganic compounds by replacing the suffix ‘ine’ with ‘ide’.
We then move on to chemical equilibrium and the factors that impact it. We study reversible reactions, which involve reactants and products that are interchangeable, before laying out the distinctive features of an equilibrium reaction under constant conditions. The course defines equilibrium expression’ and traces the connection between the equilibrium constant and temperature, pressure, concentration and catalysts. We compare weak and strong acids and bases and outline their dissociation processes. This is followed by an analysis of pH and the scale used to measure the acidity of a solution.
The course covers reaction kinetics, which is the study of the rate of a chemical reaction and the variables that affect it. We explain how the quantity of reactants or products changes over time and how that affects the rate of reaction. We also examine collision theory, which explains how reaction conditions like energy and orientation influence the rate of the reaction. Finally, we demonstrate the acceleration of a chemical reaction by raising the temperature, concentration and surface area or using a catalyst. This basic chemistry course helps learners and students understand complex chemical concepts and breaks down complicated chemical processes into easy-to-understand steps. Sign up to understand chemical reactions and the equations used to calculate them.
What You Will Learn In This Free Course
View All Learning Outcomes View Less All Alison courses are free to enrol, study, and complete. To successfully complete this Certificate course and become an Alison Graduate, you need to achieve 80% or higher in each course assessment.
Once you have completed this Certificate course, you have the option to acquire an official Certificate, which is a great way to share your achievement with the world.
Your Alison certificate is:
- Ideal for sharing with potential employers.
- Great for your CV, professional social media profiles, and job applications.
- An indication of your commitment to continuously learn, upskill, and achieve high results.
- An incentive for you to continue empowering yourself through lifelong learning.
Alison offers 2 types of Certificate for completed Certificate courses:
- Digital Certificate: a downloadable Certificate in PDF format immediately available to you when you complete your purchase.
- Physical Certificate: a physical version of your officially branded and security-marked Certificate
All Certificate are available to purchase through the Alison Shop. For more information on purchasing Alison Certificate, please visit our FAQs. If you decide not to purchase your Alison Certificate, you can still demonstrate your achievement by sharing your Learner Record or Learner Achievement Verification, both of which are accessible from your Account Settings.











 Avg. Hours
Avg. Hours  CPD Accredited
CPD Accredited 
 Total XP:
Total XP: 
 Knowledge & Skills You Will Learn
Knowledge & Skills You Will Learn 







