This advanced chemistry course delves into electrochemistry and explains electrolysis, electrode potential, cells and batteries. We begin by discussing redox reactions found in two types: oxidation and reduction reactions. We then describe the chemical reactions influenced by a passing current (called ‘electrolysis’), the electrolysis cell with its components and reactions at the anode and cathode. We then explain how to use quantitative electrolysis to determine the amount of metal deposited when passing a specific current. The course shows you how to calculate the Avogadro constant using an electrolytic method.
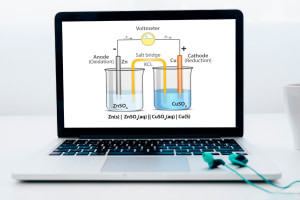
The course then moves on to electrode potential and lays out the method of measuring the electrode potential of a half-cell using a reference electrode (for example, a standard hydrogen electrode with a known potential). We also investigate galvanic cells (voltaic cells), which use spontaneous redox reactions to produce electricity, and their constituent parts. We also compare electrolytic and galvanic cells. The course then examines standard cell potential, cell voltage (standard cell potential) and how temperature, pressure or concentration affect the electrode potential.
We then cover the Nernst equation, which relates the electrode potential to the temperature and concentration of an electrode. The course investigates primary batteries (non-chargeable disposable batteries), including cylindrical and button primary batteries along with their components, and some secondary batteries (rechargeable) such as lead and Lithium-ion batteries, along with their usage in cars and electronics. We also discuss hydrogen-oxygen fuel cells that power automobiles by generating energy through a redox reaction. Finally, we outline the use of molten electrolytes and aqueous electrolytes. This advanced chemistry course suits chemistry students and aspiring electrical engineers. Sign up to learn more about batteries and electrochemistry.
What You Will Learn In This Free Course
View All Learning Outcomes View Less All Alison courses are free to enrol, study, and complete. To successfully complete this Certificate course and become an Alison Graduate, you need to achieve 80% or higher in each course assessment.
Once you have completed this Certificate course, you have the option to acquire an official Certificate, which is a great way to share your achievement with the world.
Your Alison certificate is:
- Ideal for sharing with potential employers.
- Great for your CV, professional social media profiles, and job applications.
- An indication of your commitment to continuously learn, upskill, and achieve high results.
- An incentive for you to continue empowering yourself through lifelong learning.
Alison offers 2 types of Certificate for completed Certificate courses:
- Digital Certificate: a downloadable Certificate in PDF format immediately available to you when you complete your purchase.
- Physical Certificate: a physical version of your officially branded and security-marked Certificate
All Certificate are available to purchase through the Alison Shop. For more information on purchasing Alison Certificate, please visit our FAQs. If you decide not to purchase your Alison Certificate, you can still demonstrate your achievement by sharing your Learner Record or Learner Achievement Verification, both of which are accessible from your Account Settings.











 Avg. Hours
Avg. Hours  CPD Accredited
CPD Accredited 
 Total XP:
Total XP: 
 Knowledge & Skills You Will Learn
Knowledge & Skills You Will Learn 







