

End of Month SALE! 🤩 Get 25% off certs & diplomas now!Ends in : : :
Claim your Discount!



 Teaching & Academics
Teaching & Academics Engineering & Construction
Engineering & Construction
By the end of this course, you will be able to:

By the end of this course, you will be able to:

By the end of this course, you will be able to:
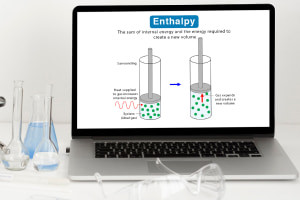
By the end of this course, you will be able to:
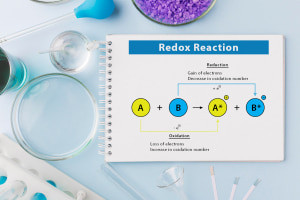
By the end of this course, you will be able to:

By the end of this coursee, you will be able to:

By the end of this course, you will be able to:

By the end of this course, you will be able to:




